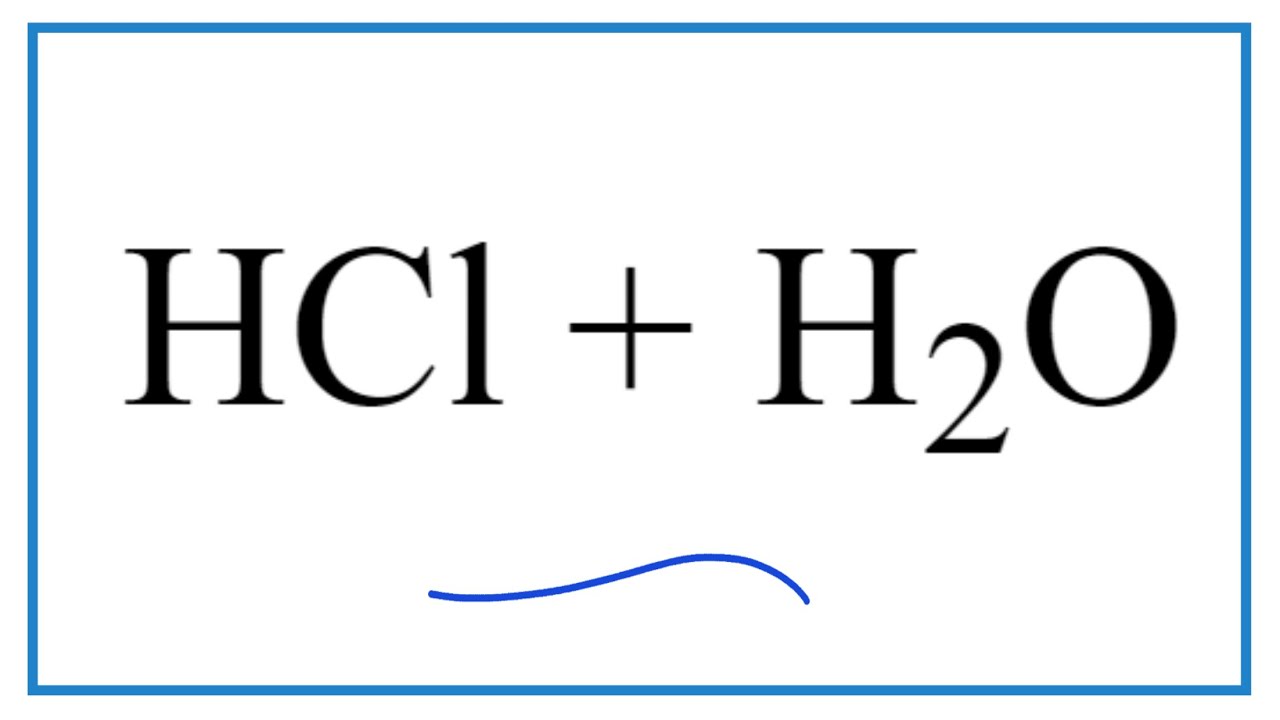Adding hydrochloric acid to water, a seemingly simple procedure, carries significant implications across various scientific and industrial domains. From adjusting pH levels in swimming pools to enabling complex chemical reactions in laboratories, understanding the nuances of this process is paramount. Mishandling this potent acid can lead to hazardous situations, emphasizing the need for a cautious and informed approach.
So, why is the order – acid to water – so crucial? The dilution of hydrochloric acid in water is an exothermic reaction, releasing a significant amount of heat. When water is added to acid, the small amount of water can rapidly boil, potentially splashing concentrated acid. Conversely, adding acid to water allows the larger volume of water to absorb the generated heat, minimizing the risk of splashing and ensuring a safer dilution process.
The history of hydrochloric acid is intertwined with the development of chemistry itself. Early alchemists experimented with mixtures containing hydrochloric acid, albeit in impure forms. The isolation and characterization of pure hydrochloric acid is often credited to Jabir ibn Hayyan, a prominent alchemist during the Islamic Golden Age. Its importance grew alongside the industrial revolution, finding applications in textile manufacturing, metal processing, and numerous other emerging industries.
Diluting hydrochloric acid correctly is fundamental to its safe and effective use. One primary concern is the potential for violent reactions if the acid is not handled properly. Incorrect dilution techniques can result in the release of corrosive fumes and dangerous splashes. Furthermore, accurate dilutions are critical for achieving desired pH levels and ensuring reproducible results in various applications. The precise concentration of the resulting solution depends on the volumes of acid and water used, highlighting the importance of accurate measurements.
Let's break down the process of adding hydrochloric acid to water. This process is commonly referred to as dilution, where a concentrated acid solution is mixed with water to reduce its concentration. The resulting solution has a lower molarity, meaning fewer acid molecules per unit volume. For example, adding 10 mL of concentrated hydrochloric acid to 90 mL of water creates a diluted solution with one-tenth the original concentration.
Benefits of proper hydrochloric acid dilution are threefold. Firstly, it enhances safety by minimizing the risk of acid splashes and corrosive fume release. Secondly, it allows for precise control over the final acid concentration, which is crucial for numerous applications, from pH adjustments to chemical synthesis. Lastly, correct dilution procedures ensure the reproducibility of experiments and industrial processes that rely on precise acid concentrations.
To safely dilute hydrochloric acid, follow these steps: 1) Wear appropriate personal protective equipment, including gloves, goggles, and a lab coat. 2) Use a well-ventilated area. 3) Slowly pour the measured amount of concentrated acid into the measured amount of water while stirring gently. 4) Never add water to acid. 5) Allow the solution to cool down before using or storing.
Advantages and Disadvantages of Diluting Hydrochloric Acid
| Advantages | Disadvantages |
|---|---|
| Increased Safety | Potential for hazard if not done correctly |
| Precise Control over Concentration | Requires accurate measurements and equipment |
| Reproducibility of Results | Disposal of diluted solutions can pose environmental concerns |
Five Best Practices: 1) Always add acid to water. 2) Use appropriate PPE. 3) Work in a well-ventilated area. 4) Stir continuously while adding acid. 5) Double-check calculations before diluting large volumes.
Five Real Examples: 1) Adjusting swimming pool pH. 2) Etching concrete. 3) Pickling steel. 4) Laboratory reagent. 5) Production of chlorides.
Five Challenges and Solutions: 1) Splashing - Solution: Slow addition and stirring. 2) Fume release - Solution: Ventilation. 3) Incorrect concentration - Solution: Accurate measurements. 4) Heat generation - Solution: Gradual addition of acid to a large volume of water. 5) Spillage - Solution: Secondary containment and spill kit.
FAQs: 1) What should I do if I spill hydrochloric acid? 2) Can I dilute hydrochloric acid with other liquids? 3) What is the molarity of concentrated hydrochloric acid? 4) How do I store diluted hydrochloric acid? 5) What are the safety precautions for handling hydrochloric acid? 6) How can I dispose of diluted hydrochloric acid? 7) Where can I buy hydrochloric acid? 8) What first aid should be administered in case of contact with hydrochloric acid?
Tips: Always label diluted solutions clearly. Double-check calculations to ensure accurate dilutions. Store hydrochloric acid and its diluted solutions away from incompatible materials.
In conclusion, adding hydrochloric acid to water, while seemingly straightforward, requires meticulous attention to detail and adherence to safety protocols. Understanding the chemical principles behind the dilution process, following best practices, and recognizing potential hazards are essential for ensuring a safe and effective procedure. The proper handling of this powerful acid not only safeguards individuals involved but also contributes to the accuracy and reliability of its numerous applications across diverse fields. Whether in a laboratory setting or an industrial application, the careful dilution of hydrochloric acid is a fundamental practice that underscores the importance of safety and precision in scientific endeavors. Remember, always prioritize safety and follow established procedures when handling this potent chemical. This cautious approach not only protects individuals but also ensures the integrity of experiments and industrial processes that rely on the precise use of hydrochloric acid.
Lilac party dresses a celebration of color and style
Conquering the loose gas cap a guide to a secure seal
Cultivating eco conscious kids inspiring action against air pollution
Mixing Hydrochloric Acid And Water - You're The Only One I've Told
Hydrochloric Acid by Bulan Laboratories Ltd Hydrochloric Acid from - You're The Only One I've Told
Hydrochloric Acid Use in Home Pools - You're The Only One I've Told
hydrochloric acid to water - You're The Only One I've Told
What is an Ion with picture - You're The Only One I've Told
Hydrochloric Acid Molecule Formula Hand Drawn Imitation Stock Vector - You're The Only One I've Told
3 Draw a pH scale and label water hydrochloric acid and sodium - You're The Only One I've Told
Chemical Equation For The Acid Ionization Of Hydrochloric Hcl In Water - You're The Only One I've Told
test carbonate infographic diagram showing royalty free image vector - You're The Only One I've Told
Hydrochloric acid sources health benefits and uses - You're The Only One I've Told
hydrochloric acid to water - You're The Only One I've Told
Hydrochloric Acid Water Flow Meter 4 Inch Electromagnetic Flowmeter - You're The Only One I've Told
Neutralization Reaction Infographic Diagram with example of - You're The Only One I've Told
HCl H2O Hydrochloric acid plus Water - You're The Only One I've Told
Hydrochloric acid solution 2 M - You're The Only One I've Told