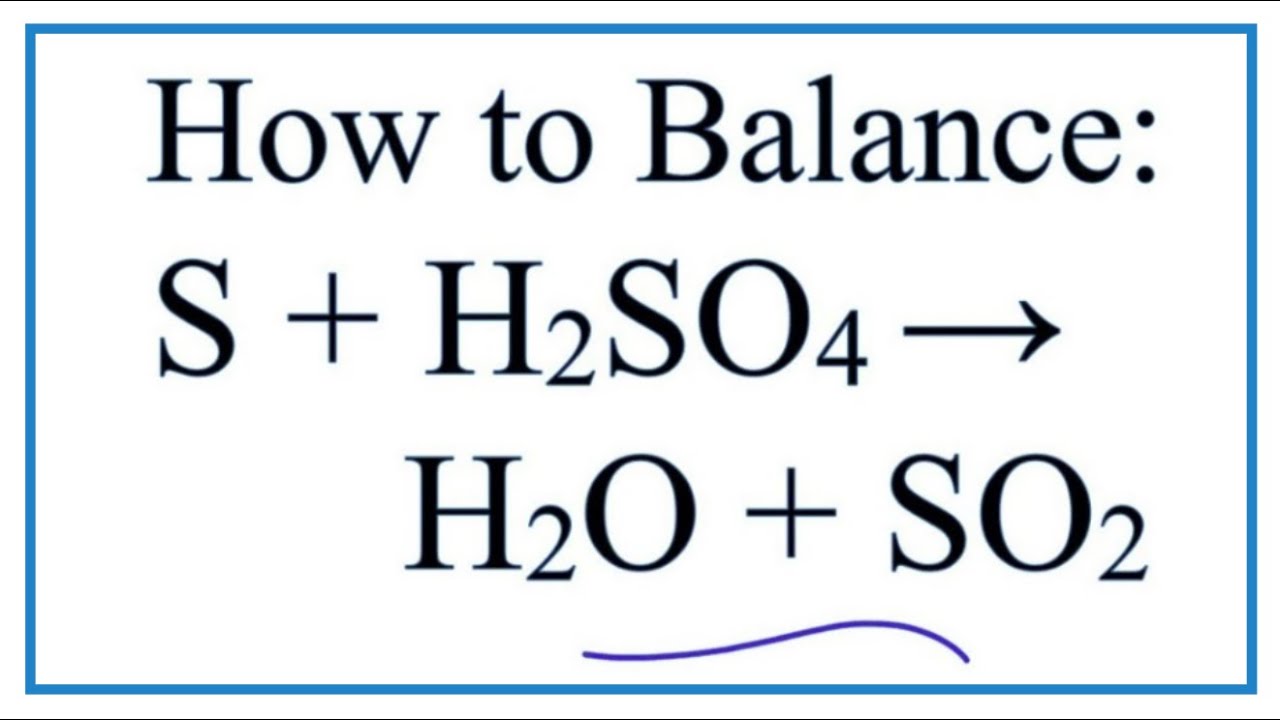
Ever wonder about the seemingly mundane white powder in your pantry or the potent acid used in industrial processes? This exploration delves into the distinct worlds of baking soda (sodium bicarbonate) and concentrated sulfuric acid, uncovering their unique properties and the surprising reactions that occur when they interact. From the kitchen to the laboratory, these substances hold a significant place in our lives, offering a wealth of applications that impact various aspects of our daily routines.
Baking soda, a staple in many households, is renowned for its leavening properties in baking. Its mild alkalinity makes it a versatile cleaning agent and a natural deodorizer. On the other hand, concentrated sulfuric acid, a highly corrosive strong acid, plays a crucial role in industrial processes, including fertilizer production, petroleum refining, and metal processing. The stark contrast in their properties makes their interaction a compelling subject of study.
The history of these two compounds is as diverse as their applications. Sodium bicarbonate, commonly known as baking soda, has been used for centuries, with ancient Egyptians utilizing naturally occurring forms for various purposes. The industrial production of baking soda began in the late 18th century. Sulfuric acid, often dubbed the "king of chemicals," boasts an even longer history. Its discovery is credited to the medieval Islamic alchemist Jabir ibn Hayyan, and its production methods have evolved significantly over time.
The significance of baking soda and sulfuric acid extends beyond their historical uses. Baking soda remains an essential ingredient in baking, contributing to the light and fluffy texture of cakes and cookies. Its mild abrasive properties make it effective for cleaning and polishing. Concentrated sulfuric acid is a vital component in the manufacture of fertilizers, detergents, and other essential products. It serves as a catalyst and dehydrating agent in numerous chemical reactions, contributing significantly to industrial processes.
Understanding the chemical nature of these compounds is crucial for their safe and effective use. Sodium bicarbonate, with its slightly alkaline pH, reacts with acids to produce carbon dioxide gas, water, and a salt. This reaction is the basis for its leavening action in baking. Concentrated sulfuric acid, a potent dehydrating agent, can react violently with water, generating significant heat. Its corrosive nature necessitates careful handling and appropriate safety precautions.
One fascinating reaction occurs when baking soda is combined with concentrated sulfuric acid. The acid neutralizes the baking soda, releasing carbon dioxide gas. This reaction demonstrates the chemical properties of both substances and highlights the importance of handling strong acids with caution.
Advantages and Disadvantages
| Substance | Advantages | Disadvantages |
|---|---|---|
| Baking Soda | Versatile, readily available, inexpensive, safe for household use | Limited effectiveness against strong stains, can leave a residue |
| Concentrated Sulfuric Acid | Essential for industrial processes, powerful dehydrating agent, strong acid | Highly corrosive, requires special handling, reacts violently with water |
Best Practices when using concentrated sulfuric acid: Always add acid to water, never water to acid. Wear appropriate personal protective equipment (PPE). Work in a well-ventilated area. Have a spill kit readily available. Dispose of waste properly.
Baking soda tips: Use as a leavening agent in baking. Mix with vinegar for a natural cleaning solution. Sprinkle in shoes to absorb odors.
In conclusion, baking soda and concentrated sulfuric acid, two seemingly disparate substances, offer unique properties and applications that contribute significantly to various aspects of our lives. From the everyday uses of baking soda in our kitchens to the crucial role of sulfuric acid in industrial processes, their impact is undeniable. Understanding their chemical nature, reactions, and safety precautions is essential for their effective and responsible use. By appreciating the distinct characteristics of these compounds, we can harness their potential while ensuring safety and minimizing risks.
Cramped quarters ditch the discomfort your guide to minimum size for a shower stall
Taking responsibility in sun valley a guide to doing your part
Unlocking the wake your guide to budget friendly wakeboard boats
Sulfuric Acid And Sugar - You're The Only One I've Told
Chemical Equation For Salt Dissolved In Water - You're The Only One I've Told
Reagent Grade Sulfuric Acid H2so4 Concentrated 38l For - You're The Only One I've Told
Answered 5 Table salt and silver nitrate - You're The Only One I've Told
SOLVED The reactions shown below were carried out during the course of - You're The Only One I've Told
Ace Ammonia And Sulphuric Acid Balanced Equation Definition Of Double - You're The Only One I've Told
baking soda and concentrated sulfuric acid - You're The Only One I've Told
Reactions of Acids and Bases - You're The Only One I've Told
Sulfuric Acid Tank Semi Trailer Caustic Soda Solution Trailer - You're The Only One I've Told
Solved Reaction 4 A reaction of baking soda with sulfuric - You're The Only One I've Told
Klabin starts up the world - You're The Only One I've Told
Household Baking Soda Concentrated Perfume Laundry Liquid Detergent - You're The Only One I've Told
Great Ammonia Plus Sulphuric Acid Balancing Equations Practice Problems - You're The Only One I've Told
Citric Acid Equation With Water - You're The Only One I've Told
baking soda and concentrated sulfuric acid - You're The Only One I've Told